Glow sticks have been a staple party accessory for decades now. No rave, festival or school disco is complete without adding a few light sticks into the mix. But have you ever thought about the science behind glow sticks? What makes glow sticks glow? What have they got to do with fireflies? And how come they’re the only source of light that’s safe to use in a catastrophe?
How is light made? Let me excite your atoms…
Light is made when an outside source of energy excites atoms and causes them to release bundles of energy called photons.
So, for example, when you switch a light bulb on, the electricity creates heat energy that causes the atoms to get all excited and speed-up. As they speed-up they collide with each other at a greater force, transferring energy to the atom’s electrons. The electrons are temporarily ‘excited’ to a higher energy level and as they return to their original level they release some of their energy in the form of light photons.
There are a variety of processes that can be used to create light, but the two you’re probably most familiar with in every-day-life are:
– Incandescence – Light is emitted due to heat. This is how fire creates light and it’s also the way your average light bulb works.
– Phosphorescence & Fluorescence – Light is emitted due to radiation energy. Its how TV screens and fluorescent light bulb create light.
Glow sticks create light in the same way – lots of excited atoms banging into each other, releasing energy in the form of light photons. But, instead of using heat or radiation, a chemical reaction is used to excite the atoms in a material. The process is called chemiluminescence.
What is chemiluminesence when it’s at home?
Chemiluminescence is one application of a natural phenomenon called luminescence. Basically, luminescence is the process of creating light without heat.
You can find it all over nature. Fireflies and glow worms use luminescence to attract mates and the Anglerfish (that big scary fish in Finding Nemo) uses it to attract prey.

Chemiluminescence recreates this luminescence found in nature by mixing multiple chemical compounds to create a chemical reaction. When the compounds mix, the atoms within them rearrange to form new compounds. The energy created by this chemical reaction results in light.
So how do glow sticks work?
In a nutshell, glow sticks give off light when two chemicals mix together. The glow stick itself is just a housing for the two chemical solutions used to create the reaction.
Most glow sticks are made-up of two separate compartments. A small, brittle inner container sits inside an outer flexible plastic container. Each of these containers holds a chemical compound.
When you bend the glow stick, the brittle inner container snaps and releases its contents to mix with the chemicals in the outer container. That’s when your atoms get all excited and start releasing energy in the form of light.

And because glow sticks use a chemical reaction within a sealed tube to create light, it makes them the safest form of light to use following a major catastrophe. In environments where the heat required for incandescent light may be dangerous, glow sticks are your go-to-guys.
So if you’re in the process of stocking your underground bunker in preparation for an apocalypse, we’ve got all the glow sticks you’ll need.
Here’s the science-y bit…
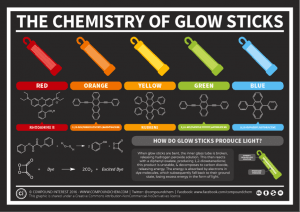
Typically, glow sticks use a chemical reaction between a hydrogen peroxide solution, and a solution that contains diphenyl oxalate and a fluorescent dye. It works like this:
- The diphenyl oxalate is oxidised by the hydrogen peroxide. This results in an unstable compound (1,2-dioxtanedione).
- The unstable compound decomposes into carbon dioxide, releasing energy into the fluorescent dye as it does so.
- The energy released causes the electrons in the dye atoms to jump to a higher level. As they return to normal levels the electrons release their energy in the form of light.
The chemical reaction is irreversible, which is why glow sticks only have one use. But you can slow down or speed-up the reaction using heat.
Warm-up a glow stick under your armpit, and the extra heat energy accelerates the reaction. It (the glow stick, not your armpit) will glow brighter, for a shorter amount of time. Stick your glow stick in the freezer and you’ll slow down the reaction. The light will dim, but the glow will last for much longer.
However quickly or slowly it happens, eventually all of the diphenyl oxalate and hydrogen peroxide are used up by the chemical reaction. When one of these compounds runs out, the glow stick will stop glowing.
What makes glow sticks glow with different colours?
A range of different chemical compounds can be used to create chemiluminescence. Depending on the chemicals used, as well as which dyes are used, the glow stick will emit different coloured light.
If you want to find out more, check out this pretty graphic by www.compoundchem.com. It shows the different chemical compounds used to create various colours of glow sticks.